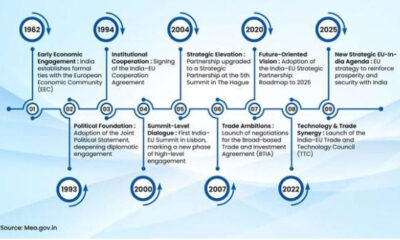
Key sentence:
- Bharat Biotech’s American accomplice, Ocugen, has gotten a suggestion from the US FDA to seek after BLA way.
- Covaxin is one of the Covid-19 antibodies being controlled under India’s inoculation program.
- All the more such authorizations are under a measure in any event 50 different nations.
Bharat Biotech’s American accomplice, Ocugen, has gotten a suggestion from the US Food and Drug Administration (FDA) to seek after Biologics License Applications (BLA) way, or full permit, for its Covid-19 immunization Covaxin, the Indian organization said in articulation on Friday.
It said the BLA way is for full endorsement rather than crisis use authorization. “All applications need to follow the BLA interaction, which is the standard cycle for antibodies,” it said.
The organization added, like this, for BLA, information from an extra clinical preliminary will be needed to help the promoting application accommodation for Covaxin. In addition, it added the cycle was probably going to broaden their general courses of events.
The organization said that no immunization produced or created in India had gotten crisis use endorsement or full licensure from the FDA.
Also read: What-the-world-needs-China-to-reveal-in-the-wuhan-lab-spill-test.
It added, along these lines, it will be an “incredible jump forward” for immunization advancement and assembling in India if Covaxin is endorsed in the UN.
As the Covid-19 cases have been decreasing in the US due to great group insusceptibility and the vaccination of a critical level of the populace, the FDA has declared no new crisis use authorization would be endorsed for new immunizations.
Covaxin is one of the Covid-19 antibodies being controlled under India’s inoculation program. It has gotten crisis use authorization from 14 nations.
All the more such authorizations are under a measure in any event 50 different nations.
Covaxin, which has been co-created by the Indian Council of Medical Research, is an entire virion inactivated antibody.
Inactivated immunizations contain infections with obliterated hereditary material. As a result, they can’t taint and trigger an insusceptible reaction.
Bharat Biotech has collaborated with Ocugen for the commercialization of the Covaxin antibody in the US. On June 3, Bharat Biotech reported that they had changed their co-advancement, supply, and commercialization consent to extend Ocugen’s selective region to popularize Covaxin to likewise incorporate Canada.
In a proclamation prior, Krishna Ella, administrator and overseeing chief, Bharat Biotech, said Covaxin had shown a superb wellbeing record in human clinical preliminaries and antibody organization under crisis use in India.
“Our objective for all immunizations created at Bharat Biotech is to give worldwide access. With its expected viability against different existing and arising variations, we accept that Covaxin is a significant antibody for everybody, including youngsters, because of its extraordinary yet conventional immunization stage.
We are perseveringly working with Ocugen to carry Covaxin to the US market and now to the Canadian market.”